It is important to maintain clear red lines for risks that are catastrophic in their impact, but have a very low probability. These can be easily overlooked because the probability, and therefore the risk, is seen as negligible. As time passes and the adverse event associated with the risk does not occur, the perception of the risk diminishes further. This situation is known as 'drift' and can result in major adverse events.
An example of this was the Northwick Park drug trial in 2006. This was the first trial of a potential drug in humans in which the six volunteers suffered terrible side effects, including catastrophic system organ failure and swelling of the brain, due to an unexpected adverse drug reaction. All six volunteers were given a dose of the drug consecutively within a 20-minute period. The protocol for the trial had been approved by the MHRA and the ethics committee.
Five minutes after the last volunteer had been given their dose, the first volunteer started to complain of a headache and side effects quickly followed. There had been a drift in the standards around the length of time between dosing volunteers.
Much earlier standards had required a period of an hour between the administration of a dose to the next volunteer. While this seems logical, no adverse event like this had occurred over a number of years and so the perception of the magnitude of this risk had reduced over time.
When an adverse event did happen, the outcome was catastrophic. This same attitude to risk is probably what allowed the financial markets to get into the terrible state we have experienced over the past few years.
Of course, this can also apply to pharmacy. What processes have you allowed to drift because no adverse effects have arisen and you therefore assume that your practices are safe? This not only applies to clinical governance, but also to financial matters, health and safety, information and leadership.
Keeping a risk log
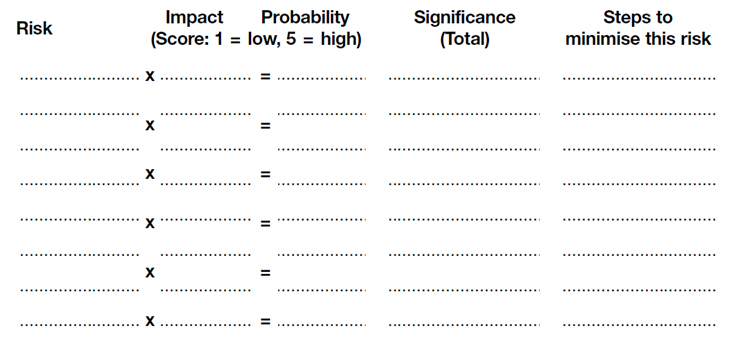
While it is important to undertake thorough risk assessments and have plans in place to reduce risks, it is also important to document this. This allows you to review and audit your risk management processes.
It also provides good evidence that you have taken all reasonable steps to reduce the risk should an adverse event occur. For health and safety risks, a risk log is a legal requirement for businesses with more than five employees, and is recommended for all businesses.
Activity
Assessing risk
- Look at a specific area of your pharmacy business (e.g. dispensing for nursing homes, the medicines counter or a specific service).
- Record the risks that you can identify in that area. Create a risk register to identify the potential impact and probability of adverse events. Identify and record what you will do to manage those risks.
- Review the health and safety risks in your pharmacy. Check that the risk reduction processes for these risks are up to date and are being implemented.
The next step is to put these ideas into action. Finish by recording your learning outcomes.
