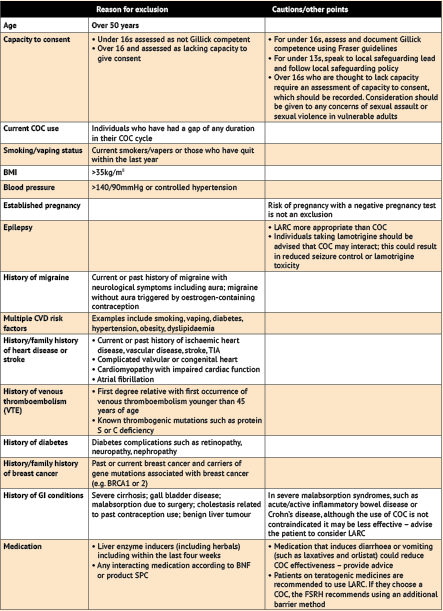
Inclusion and exclusion criteria
Pharmacy provision is more tightly defined than general prescribing guidelines in terms of access criteria. The following criteria apply to COC provision under England’s tier 1 Pharmacy Contraception Service, which applies if the medicine has been previously initiated in primary care or a sexual health clinic. There is little indication that further services – whether the tier 2 service in England, which allows initiation in pharmacy settings, or expanded services in other UK home countries – will deviate far from this.
It is worth keeping in mind that the process of reauthorising a COC is different to its conventional supply in terms of the clinical check – almost a halfway house between prescribing and dispensing. As and when COC initiation comes in, the clinical check and supply process will be different again, so it is sensible to think carefully now about what this might involve.
From the outside, the outcome is the same – provision of a COC – but much more will be involved in getting to this point. Putting in place appropriate SOPs or other protocols will be essential to underpin the various means of provision.
Inclusion criteria for reauthorising COCs
An individual presenting for ongoing supply of oral contraception:
- Previous and current supply of the COC should be established and there are no brand restrictions, although local formularies and restrictions should be referred to
- Age falls between menarche and up to, and including, 49 years
- Confirm BMI and blood pressure prior to supply (this can be self-reported by the patient).
See table below for exclusion criteria.
Exclusion criteria
Note: the list of exclusions below is lengthy but it is worth bearing in mind that for reauthorisation of supply the patient will already be taking the COC and will have been assessed prior to its initiation by the prescriber.
Note: the option of LARC should be discussed with all individuals, but particularly those for whom pregnancy is regarded as unacceptable. If the patient decides to pursue LARC, they should be signposted to an appropriate provider. Anyone who is excluded from or declines treatment should have the reasons documented and be provided with information about further options and signposting as relevant.